We are focused on your marketing authorisation success of generic medicinal products
- Global best practices with delivery capabilities
- Quality & validity of bioequivalence trials
- Trial data submitted in the marketing authorisation dossiers are of high quality, address safety issues and are verifiable.
Our Specialty
We specialise in conducting and analysing in vivo bioequivalence studies to support your marketing authorisation application for generic medicinal products.
Expertise & Experience
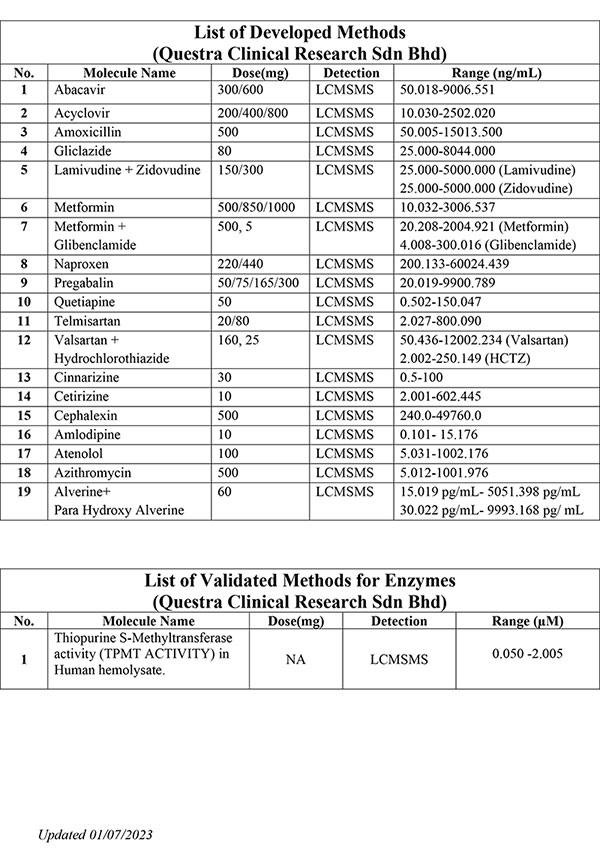
We offer expertise and experience in bioanalytical method development, method validation and quantitative sample analysis for both small and large molecules.
One-Stop Centre
Our clinical facility and bioanalytical laboratory are conveniently located on the same site, this providing efficient and more timely process for the development and marketing of generic medicinal products.
Best Practices
Our Research Centre complies with ICH-GCP guidelines, GLP principles and good practices for quality control laboratories to ensure integrity and traceability of data.
End-to-End Bioanalytical Solutions
We offer end-to-end bioanalytical solutions from protocol design and project management to final study report submissions (eCTD/ ICH E3 compliant dossier submission).
Our End-to-End BA/ BE Services, Customizable to Your Needs
- Study design
- Protocol development
- Bioanalytical method development & validation
- Informed Consent Form (ICF) development
- Case Report Form (CRF) design
- High quality ethics application & review
- Clinical trial registration
- Clinical Trial Import License (CTIL)/ Clinical Trial Exemption (CTX) application
- Recruitment & selection of subjects
- Study sample analysis
- Pharmacokinetic & statistical calculations
- Final study report
- Marketing authorization dossier submission
- Archival of study materials
- Project management